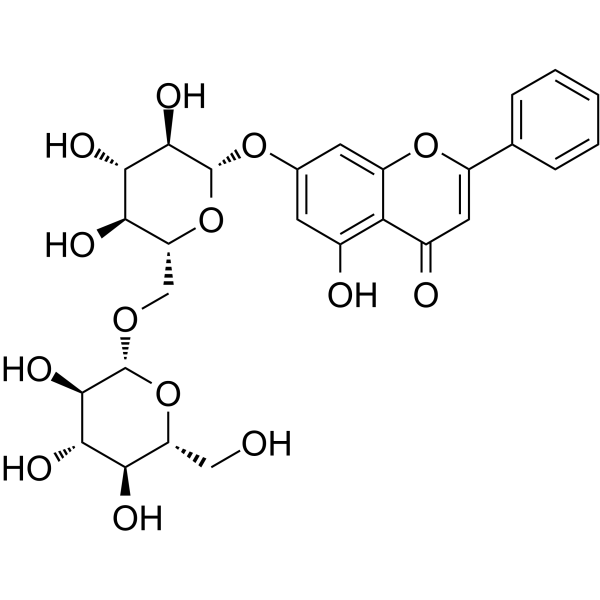
Chrysin 7-O-beta-gentiobioside
CAS No. 88640-89-5
Chrysin 7-O-beta-gentiobioside( Chrysin 7-O-β-gentiobioside )
Catalog No. M28253 CAS No. 88640-89-5
Chrysin 7-O-beta-gentiobioside, a glycosylation product of Chrysin, is from Spartium junceum.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 205 | Get Quote |


|
| 10MG | 329 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameChrysin 7-O-beta-gentiobioside
-
NoteResearch use only, not for human use.
-
Brief DescriptionChrysin 7-O-beta-gentiobioside, a glycosylation product of Chrysin, is from Spartium junceum.
-
DescriptionChrysin 7-O-beta-gentiobioside, a glycosylation product of Chrysin, is from Spartium junceum.
-
In Vitro——
-
In Vivo——
-
SynonymsChrysin 7-O-β-gentiobioside
-
PathwayOthers
-
TargetOther Targets
-
RecptorCOX
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number88640-89-5
-
Formula Weight578.52
-
Molecular FormulaC27H30O14
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESOC[C@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3cc(O)c4c(c3)oc(cc4=O)-c3ccccc3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Obach RS, et al. In vitro metabolism and covalent binding of enol-carboxamide derivatives and anti-inflammatory agents sudoxicam and meloxicam: insights into the hepatotoxicity of sudoxicam. Chem Res Toxicol. 2008 Sep;21(9):1890-9.
molnova catalog



related products
-
Nocistatin
Nocistatin
-
AZD0780
AZD0780 (EX-A6975) is an oral small molecule PCSK9 inhibitor being developed by AstraZeneca as a first-in-class treatment for patients with dyslipidemia that is uncontrolled with statins alone.
-
16-hydroxy Hexadecan...
16-hydroxy Hexadecanoic Acid (Juniperic acid) is a compound isolated from the leaves and stems of Arabidopsis thaliana and can be used to prevent, alleviate, or treat metabolic diseases.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


